

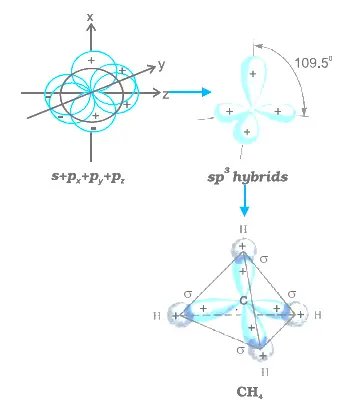
In sp2 the orient to form a triangle making an angle of 120 degree ,in sp3 the orient to form a tetrahedron making an angle of 109 degree 28 minute ,in sp3d the orient to form triagonal bipyramidal where the angle between axial bond is 180 degree and between equitorial bond is 120 degree and between axial and equitorial it is 90 degree. Trigonal planar: Three electron groups involved resulting in sp2 hybridization, the angle between the orbitals is 120°. The geometry of the orbital arrangement: Linear: Two electron groups involved resulting in sp hybridization, the angle between the orbitals is 180°. What is the geometry of the orbital arrangement in SP2? To bond six fluorine atoms, the 3 s orbital, the three 3 p orbitals, and two of the 3 d orbitals form six equivalent sp3d2 hybrid orbitals, each directed toward a different corner of an octahedron. How many sp3d and sp3d2 hybrid orbitals are there? There will be a vacant 3d orbital if 4s, 4p and 4d orbitals are filled with lone electron pairs drawn from six chloride ligands. Sp3d2 (nd orbitals are involved outer orbital complex or high-spin or spin-free complex)Īs per VB Theory of complex compounds, in 3- Mn is said to undergo sp3d2 hybridisation forming outer orbital complex. Which d orbitals are involved in sp3d2 and d2sp3 hybridization respectively? Coordination number How many 90 degree bond angles are in PCl5? These hybrid orbitals are arranged in an octahedral geometry. This hybridization results in six hybrid orbitals. What is d2sp3 Hybridization? d2sp3 hybridization is the mixing of s and p atomic orbitals of the same electron shell with d orbitals of another electron shell to form d2sp3 hybrid orbitals. sp 3 hybrid orbitals are oriented at bond angle of 109.5 o from each other. Hybridization of an s orbital with all three p orbitals (p x, p y, and p z) results in four sp 3 hybrid orbitals. What is the angle between sp3 and sp3 hybrid orbitals? The key difference between sp3d2 and d2sp3hybridization is that, sp3d2 hybridization involves atomic orbitals of same electron shell whereas d2sp3 hybridization involves atomic orbitals of two electron shells. Summary – sp3d2 vs d2sp3 Hybridization These are different in many ways.
What is the difference between sp3d2 and d2 sp3?
These six sp3d2 orbitals are arranged in octahedral symmetry by making 90° angles to each other. Intermixing of one ‘s’, three ‘p’ and two ‘d’ orbitals of almost same energy by giving six identical and degenerate hybrid orbitals is called sp3d2 hybridization. What angle exists between orbitals in sp3d2 hybrid orbitals?


 0 kommentar(er)
0 kommentar(er)
